Push–pull unsymmetrical substitution in nickel(ii) complexes with tetradentate N2O2 Schiff base ligands: synthesis, structures and linear–nonlinear optical studies†
Abstract
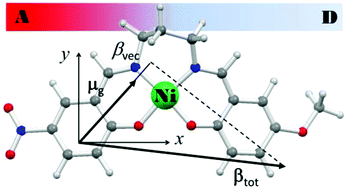
New push–pull (A–D) nickel(II) compounds of general formula [Ni(5-A-5′-D-saltn)] (3a–3l) with unsymmetrically-substituted N2O2 tetradentate Schiff base ligands are reported here. The ligands 5-A-5′-D-saltn2− (H2saltn = N,N′-bis(salicylidene)diaminopropane) possess differently-substituted salicylaldehyde (A/Dsal) moieties condensed to 1,3-diaminopropane (tn), and carry either an electron acceptor (A = H, Br, or NO2) or donor (D = H, Me, or OMe) group in the para position with respect to the coordinated phenoxido oxygen atoms. These compounds could be obtained by template synthesis involving derivatives [Ni(Gsal)2(H2O)2], 1a–e (G = NO2, Br, H, Me and OMe, respectively) and [Ni(GL)2], 2a–d (GL− = (E)-2-((3-aminopropylimino)methyl)-4-G-phenolate, G = NO2, Br, H and Me, respectively). Scrambling of the ligands and condensation to compounds 3 was suitably achieved by refluxing compounds 1 and 2 that carry the G groups suitable for the desired final A–D combination. Dinuclear intermediates [Ni2(μ-GL)2(G′sal)2] (4a,b,e,f,g) were also detected and isolated. The single-crystal X-ray diffraction structures of [Ni(5′-OMe-saltn)]·CHCl3 (3c·CHCl3), [Ni(5-Br-5′-OMe-saltn)]·EtOH (3g·EtOH) and [Ni(5-NO2-saltn)] (3j) show different degrees of distortion around the central core, leading to saddle-like (3c), planar (3g) and step-like (3j) molecular conformations. DFT geometrical optimization of the compounds showed that, for isolated molecules, the saddle-like conformation is slightly more stable with respect to the other conformations. UV-visible absorption spectra showed structured absorption profiles at about 320–440 nm, whose intensity was amplified by the presence of the nitro group, and this was assigned to a convolution of one metal-to-ligand charge transfer and two intra-ligand charge transfer transitions by TDDFT computations. Surprisingly, UV-visible spectra of the derivatives with Br were comparable to the derivatives with Me, suggesting that in this case the behaviour of the halogen was as a weak electron donor group. The experimental investigation (through electric-field-induced second-harmonic and solvatochromic measurements) of the second-order NLO responses of compounds 3, in conjunction with the theoretical calculations, revealed that the observed NLO efficiency is determined by the combined effect of two almost orthogonal charge transfer directions within the molecules, one along the axis approximately bisecting the donor and the accepting moieties and the other along the A–D axis.


 Please wait while we load your content...
Please wait while we load your content...