On solvated tin(iv) ions and the coordination chemistry of high-valent d10 metal ions†
Abstract
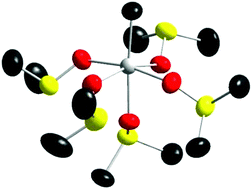
A very slow oxidation of dimethylsulfoxide (dmso) solvated tin(II) ions in solution results in the formation of a crystalline, structurally determined compound, [CH3Sn(OS(CH3)2)5](ClO4)3, whereas a similar reaction in N,N-dimethylthioformamide (dmtf) forms a crystalline solid with a proposed binuclear [Sn2(SH)2(SCHN(CH3)2)8]6+ entity but whose exact formula remains undetermined. Both solids precipitate with time in their respective mother liquids and constitute the first two tin(IV) and even tetravalent d10 metal ion solvate complexes ever reported. An EXAFS study showed that the structure of the [CH3Sn(OS(CH3)2)5]3+ complex is identical in solid state and dmso solution. While the exact chemical reaction pathways are unknown, the formation of these complexes constitute a novel way of obtaining solvated tin(IV) ions in standard, commonplace organic media.


 Please wait while we load your content...
Please wait while we load your content...