Tetranuclear MnII, CoII, CuII and ZnII grid complexes of an unsymmetrical ditopic ligand: synthesis, structure, redox and magnetic properties†
Abstract
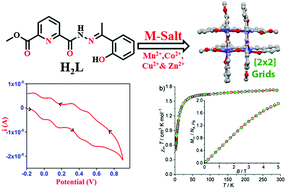
Synthesis towards the preparation of an unsymmetrical binucleating Schiff-base ligand, (H2L) is discussed. It has been synthesized by reacting methyl 6-(hydrazinecarbonyl)picolinate with 2-hydroxy acetophenone. The ligand (H2L) comprises two different asymmetric binding pockets; however, when reacted with Mn(II), Co(II), Cu(II) and Zn(II) salts, very stable self-assembled [2 × 2] grid complexes form regardless of the employed metal-to-ligand ratio. The obtained complexes [Mn4L4]·(CH3CN) (1), [Co4L4]·(CHCl3) (2), [Cu4L4]·(CHCl3) (3) and [Zn4L4] (4) have been fully characterized by physicochemical methods, including ESI mass spectrometry and X-ray crystallographic analyses, and their EPR, magnetic and redox properties are discussed. All discussed complexes self-assembled in a ‘head-to-tail’ fashion leading to [2 × 2] grid architectures. Mn, Co and Cu grid complexes show weak to moderate antiferromagnetic coupling among the four metal centers. The high stability of the grid structures is in line with the lack of any observable dissociation or exchange between metal ions in solution. Complexes 1 and 2 show four quasi-reversible to irreversible oxidative responses in cyclic voltammograms at a glassy carbon working electrode in 1,2-dichlorobenzene and at a platinum working electrode in dichloromethane, respectively.


 Please wait while we load your content...
Please wait while we load your content...