4-Halo-1,2,3-triazolylidenes: stable carbenes featuring halogen bonding†
Abstract
Synthesis and coordination of 4-halo-1,2,3-triazolylidenes have been developed. These novel ligands featured the character with σ-donation at carbon and a σ-hole at the halogen. Halogen bonding was observed by single crystal X-ray diffraction in their coinage metal complexes. The electronic properties of 4-iodo-1,2,3-triazolylidene were studied by both Ir–CO frequencies of the Tolman electronic parameter (TEP) and Huynh's electronic parameter (HEP) method, which suggested similar electronic properties to those of imidazolylidenes. During HEP tests, an interesting tunability was observed when different electron donors were employed.
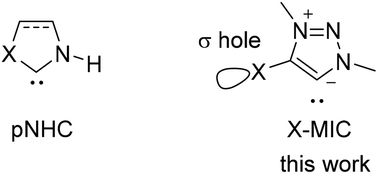


 Please wait while we load your content...
Please wait while we load your content...