Reactivity of a gold(i)/platinum(0) frustrated Lewis pair with germanium and tin dihalides†
Abstract
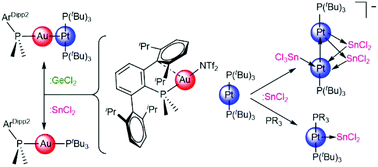
The reactivity of germanium and tin dichlorides with a transition metal-only frustrated Lewis pair based on Au(I) and Pt(0) compounds bearing bulky phosphine ligands is described in this work. We have examined both the reactivity of tetrylene dihalides towards the individual components of the metallic pair, as well as under metal/metal cooperative conditions. These studies allowed us to isolate several uncommon homo- and heterometallic structures. Computational methods have been employed to investigate the bonding scheme of one of these highly-reduced metallic aggregates. In addition, we have developed a tin-promoted strategy to access heteroleptic diphosphine platinum(0) compounds.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...