Aryl C–O oxidative addition of phenol derivatives to nickel supported by an N-heterocyclic carbene via a Ni0 five-centered complex†
Abstract
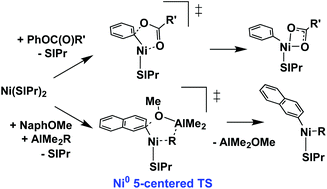
Phenol derivatives have been increasingly used as alternatives for aryl halides in cross-coupling reactions due to their lower toxicity and easier availability. Nickel complexes have been developed as efficient catalysts for aryl C–O bond activation of phenol derivatives. Herein, we performed a density functional study to investigate the aryl C–O bond oxidative addition of phenol derivatives on Ni-SIPr. For phenyl sulfonates and phenyl esters, the pathway via a five-centered transition state involving the interaction from sulfonyl/carbonyl O to Ni is preferred over that via a three-centered transition state. While the five-centered transition state with the interaction from sulfamoyl N to Ni is favored over the interaction from sulfamoyl O to Ni for phenyl sulfamate, the interaction from carbamoyl O to Ni is more favored for phenyl carbamate. For aryl ethers, the Lewis acid AlMe3 facilitates aryl C–O bond activation by forming a Lewis acid/base adduct with 2-methoxynaphthalene (NaphOMe) resulting in a lower free energy barrier than that in the absence of AlMe3. While the free energy barriers for the aryl C–O bond oxidative addition of a NaphOMe/AlMe2R (R = Me, Ph, OMe, and H) adduct via the “classical” three-centered transition state are all similar, the corresponding free energy barriers via the five-centered transition state, involving the interaction from the R group of AlMe2R to Ni, can be lower and depend on the R group. Not only is the aryl C–O bond weakened, but the nucleophilicity of Ni is enhanced in the latter pathway. In fact, these key interactions are analogous to those via the five-centered transition states of phenyl sulfonate/sulfamate and phenyl ester/carbamate. Our results revealed that the pathway involving an additional electron donating interaction to Ni via the five-centered transition state facilitates the aryl C–O bond oxidative addition of phenol derivatives. Through this pathway, the appropriate use of organoaluminum can improve the efficiency of Ni catalysts for cross-coupling reactions of inert aryl ethers.


 Please wait while we load your content...
Please wait while we load your content...