Regulating effect of heterojunctions on electrocatalytic oxidation of methanol for Pt/WO3-NaTaO3 catalysts†
Abstract
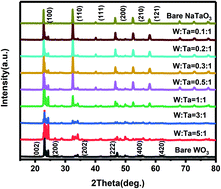
Pt/WO3-NaTaO3 composite catalysts for different W/Ta molar ratios were obtained via a facile hydrothermal method. The WO3-NaTaO3 heterojunction was constituted and could be regulated by the adjustment of the W : Ta ratio, as confirmed by multiple characterizations. Due to the favorable CO anti-poisoning characteristics ascribed to the sufficient surface –OHad provided by both WO3 and NaTaO3, excellent stability of the WO3-NaTaO3 composite in acidic and alkaline environments, and charge transfer from metal oxide to Pt, the as-obtained Pt/WO3-NaTaO3 composite catalyst could exhibit desirably high electrocatalytic performances. As a result, the as-prepared Pt/WO3-NaTaO3 (W : Ta = 3 : 1) and Pt/WO3-NaTaO3 (W : Ta = 0.2 : 1) products exhibited optimal performances in the methanol oxidation reaction (MOR) process in acids and alkalis, respectively, as compared to those of commercial Pt/C, Pt/NaTaO3, and Pt/WO3 catalysts. In particular, in acidic and alkaline environments, the highest electrocatalytic activity of Pt/WO3-NaTaO3 catalyst could reach three times that of commercial Pt/C and its stability could reach more than five times that of commercial Pt/C. Density functional theory calculations further proved the enhancement of WO3 with regard to methanol electrooxidation.


 Please wait while we load your content...
Please wait while we load your content...