A first phosphine oxide-based extractant with high Am/Cm selectivity†
Abstract
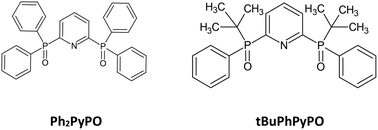
A new phosphine oxide ligand demonstrates high selectivity for the Am–Cm pair with SF = 2.9–3.5 and the Am–Eu pair with SF = 7.3–8.5 in a range of 0.1–3 M nitric acid. Thermodynamic measurements show that the entropy factor is responsible for selectivity observed in the extraction experiment. The most prevalent complexes of all three metal ions were (Ph2PyPO)2M(NO3)3. According to their DFT modelling, the M–N distances for the Cm ion were larger than those for Am, so the last ion enters deeper into the pseudo-cavity of the ligand, which causes the observed selectivity.


 Please wait while we load your content...
Please wait while we load your content...