Sodium inhibits the formation of ammonium-substituted solid solutions of octacalcium phosphate by filling its substitution site
Abstract
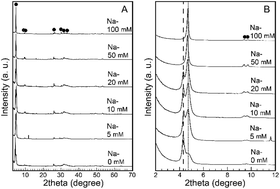
Octacalcium phosphate (OCP) is a layered type of calcium phosphate that shows promise for pharmaceutical and biomedical applications because it offers both excellent biocompatibility and a unique, robust crystal structure that readily accepts substitution by various molecules. Although several cations can be incorporated into the OCP crystal lattice by ionic substitution, little is known about the relative probability of different ions to substitute into the OCP crystal lattice. In this study, we focus on Na and NH4, which are known to enter the OCP crystal lattice by ionic substitution. We investigate which of these two is most likely to substitute into OCP in a system containing both ions. In this work, OCP is fabricated from monocalcium phosphate monohydrate via hydrolysis in a solution containing 1 mol L−1 (NH4)2HPO4 with NaCl concentrations ranging from 0 to 100 mmol L−1. X-ray diffraction and thermal analyses indicate that the structure characteristic of NH4 ionic substitution in OCP is attenuated as the Na concentration increases. Furthermore, when the Na concentration exceeds 50 mmol L−1 (Na/NH4 ≥ 1/20), the structure characteristic of NH4 ionic substitution into OCP almost completely disappears. These results indicate that Na is more likely to be ionically substituted into the OCP crystal structure than NH4 and thereby inhibits the structural change of OCP caused by ionic substitution of NH4.


 Please wait while we load your content...
Please wait while we load your content...