A pentadentate member of the picolinate family for Mn(ii) complexation and an amphiphilic derivative†
Abstract
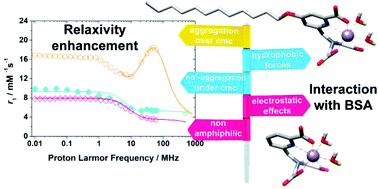
We report a pentadentate ligand containing a 2,2′-azanediyldiacetic acid moiety functionalized with a picolinate group at the nitrogen atom (H3paada), as well as a lipophylic derivative functionalized with a dodecyloxy group at position 4 of the pyridyl ring (H3C12Opaada). The protonation constants of the paada3− ligand and the stability constant of the Mn(II) complex were determined using a combination of potentiometric and spectrophotometric titrations (25 °C, 0.15 M NaCl). A detailed relaxometric characterisation was accomplished by recording 1H Nuclear Magnetic Relaxation Dispersion (NMRD) profiles and 17O chemical shifts and relaxation rates. These studies provide detailed information on the microscopic parameters that control their efficiency as relaxation agents in vitro. For the sake of completeness and to facilitate comparison, we also characterised the related [Mn(nta)]− complex (nta = nitrilotriacetate). Both the [Mn(paada)]− and [Mn(nta)]− complexes turned out to contain two inner-sphere water molecules in aqueous solution. The exchange rate of these coordinated water molecules was slower in [Mn(paada)]− (k298ex = 90 × 107 s−1) than in [Mn(nta)]− (k298ex = 280 × 107 s−1). The complexes were also characterised using both DFT (TPSSh/def2–TZVP) and ab initio CAS(5,5) calculations. The lipophylic [Mn(C12Opaada)]− complex forms micelles in solution characterised by a critical micellar concentration (cmc) of 0.31 ± 0.01 mM. This complex also forms a rather strong adduct with Bovine Serum Albumin (BSA) with an association constant of 5.5 × 104 M−1 at 25 °C. The enthalpy and entropy changes obtained for the formation of the adduct indicate that the binding event is driven by hydrophobic interactions.


 Please wait while we load your content...
Please wait while we load your content...