Synthesis, structure, docking and cytotoxic studies of ferrocene–hormone conjugates for hormone-dependent breast cancer application†
Abstract
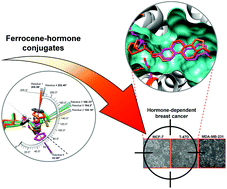
Previously, ferrocene incorporation into the principal structural component of biologically active molecules resulted in enhanced cytotoxic activity against hormone-dependent MCF-7 and T-47D and hormone-independent MDA-MB-231 breast-cancer cell lines. Here we explore 4 new ferrocene estrogen conjugates at position 16 of the estrogen hormone and compared them to the previously reported ferrocene estrogen conjugate 3-ferrocenyl-estra-1,3,5(10)-triene-17β-ol. The ferrocene conjugate 16-ferrocenylidene-3-hydroxyestra-1,3,5(10)-trien-17-one was synthesized using estrone and ferrocene carboxaldehyde as starting materials in 86% yield. This ferrocene complex was used as the starting material for the synthesis of new ferrocene estrogen conjugates by a short linker group at position 16 of the estrogen hormone. The position and stereochemistry of the linker was verified by its crystal structure. The ferrocene redox behavior, in vitro studies on breast-cancer cell lines and docking studies on ERα are presented. The data suggest that the ferrocene conjugates presented, either at position 3 or 16 of the estrogen, could serve as vectors and can be recognized by ERα as a delivery mechanism into the cell. These new ferrocene hormone conjugates showed cytotoxic activity comparable to that of conventional therapeutic drugs such as tamoxifen and cisplatin.
- This article is part of the themed collection: Bioinspired reactivity and coordination chemistry


 Please wait while we load your content...
Please wait while we load your content...