Towards coupling direct activation of methane with in situ generation of H2O2
Abstract
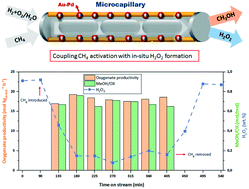
Due to the explosive nature of H2, O2 and CH4 mixtures, the concept of coupling in situ synthesis of H2O2 with low-temperature single-step methane conversion to methanol has not received sufficient attention. This study aimed to investigate this process using a microchannel reactor, which offers the opportunity to explore the process under a wide range of concentrations. Direct methane activation with in situ generation of H2O2 was successfully demonstrated in a microcapillary containing Au–Pd nanoparticles embedded on its silica-coated walls. The effect of H2, O2 and CH4 partial pressures, H2/O2 molar ratio, gas-to-liquid (G/L) ratio and liquid phase weight-hourly-space-velocity (WHSV) on the productivity and product distribution was investigated. CH4 partial pressure had the most significant effect on the productivity, while H2 and O2 partial pressures influenced the productivity less. The methane activation rate was found to be correlated with the H2O2 formation rate. With only O2 or pre-formed stabilized H2O2 methane activation was not found, in situ H2O2 synthesis was therefore essential. G/L affected neither the product distribution nor the productivity, however, lowering WHSV altered the product distribution favoring methanol formation.


 Please wait while we load your content...
Please wait while we load your content...