Interplay of the adsorption of light and heavy paraffins in hydroisomerization over H-beta zeolite†
Abstract
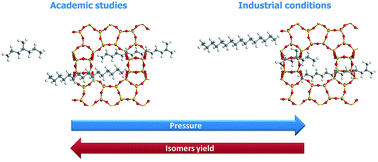
The effect of pressure on the activity and selectivity of well-balanced Pt/H-beta zeolite bifunctional catalysts in n-hexadecane (n-C16) hydroisomerization was studied. The turnover frequency per Brønsted acid site of the catalyst decreased when the total pressure was increased due to the lower concentration of olefins at equilibrium, in line with the classical bifunctional mechanism. Conversely, when the total pressure was increased, the C16 isomer yield unexpectedly decreased in contradiction with the pure kinetic/thermodynamic effect of olefin pressure on the reaction rates. Thanks to grand canonical Monte Carlo (GCMC) calculations, non-idealities in adsorption behavior in the zeolite micropores were revealed when a representative reaction medium was considered. Via mechanistic kinetic simulations combined with GCMC simulations for the relevant intermediate concentrations, the pressure effect on catalyst selectivity is proposed to be due to the interplay between the (light) cracked products and the (heavy) hexadecanes in the pores of beta zeolite which leads to a pressure dependency on the adsorption behavior.


 Please wait while we load your content...
Please wait while we load your content...