Molecular design of chiral zirconium metal–organic frameworks for asymmetric transfer hydrogenation of imines†
Abstract
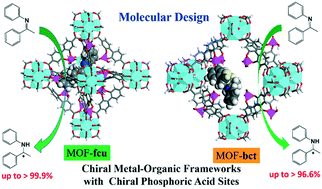
Chiral metal–organic frameworks (MOFs) have been considered as intriguing heterogeneous catalysts for asymmetric transformations. Herein, we computationally design two chiral zirconium MOFs (MOF-fcu and -bct) by assembling highly stable Zr-clusters with phosphoric acid derived organic linkers. The catalytic performance of the two MOFs is evaluated for the asymmetric transfer hydrogenation (ATH) of N,1-diphenylethan-1-imines using density functional theory (DFT) calculations. On both MOFs, the transition states (Z)-syn-TS-R and (E)-syn-TS-S are found to interact with the chiral active sites through H-bonds, (Z)-syn-TS-R possesses a lower Gibbs energy than (E)-syn-TS-S, and the pathway via(Z)-syn-TS-R is kinetically and thermodynamically favored. The enantiomeric excess (ee) values to form (R)-/(S)-amines are predicted to be 99.9% on MOF-fcu and 96.6% on MOF-bct, respectively. By contrast, the ee is zero in the kinetically favored pathway for ATH catalyzed by a chiral phosphoric acid (L1A2). The remarkably enhanced enantioselectivity on the MOFs is attributed to the steric hindrance and confinement effect of the framework cavity. Particularly, the 12-connected octahedral cavity in MOF-fcu offers better confinement than the 10-connected octahedral cavity in MOF-bct; thus MOF-fcu exhibits a higher ee value than MOF-bct. Furthermore, three solvents (toluene, dichloromethane and acetonitrile) are examined for ATH. With decreasing solvent polarity, the activation barrier drops while the enantioselectivity remains nearly unchanged. Among the three solvents, toluene appears to be the best solvent. This computational study provides microscopic insight into the mechanism of imine transformation on chiral MOFs and highlights the crucial role of cavity size and shape in the transformation. The two designed chiral MOFs with highly enantioselective performance might be interesting candidates for the ATH of imines.


 Please wait while we load your content...
Please wait while we load your content...