Enhancing electrostatic interactions to activate polar molecules: ammonia borane methanolysis on a Cu/Co(OH)2 nanohybrid†
Abstract
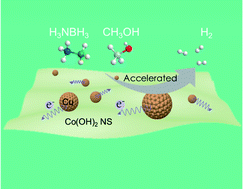
Optimization of metal–support interactions (MSIs) is at the core of heterogeneous catalyst design. For polar reactants, electrostatic interactions resulting from MSIs can facilitate their activation. In this work, a feasible in situ method has been employed to control the electrostatic properties at the interface of a noble-metal-free Cu/Co(OH)2 nanohybrid catalyst. On the Cu/Co(OH)2 interface, the positively charged copper enhances the polar molecule adsorption. By varying the metal/support ratio, a highly efficient catalytic activity for the methanolysis of ammonia borane (AB) with an initial turnover frequency (TOF) of 61.63 mol(H2) mol(catalyst)−1 min−1 and long-term stability at ambient temperature were observed. Theoretical analysis unravels the role of charge transfer in promoting the reactions and the metal/support ratio in manipulating the catalytic activity via tuning electrostatic interactions.
- This article is part of the themed collection: Catalysis Science & Technology 10th Anniversary Symposium


 Please wait while we load your content...
Please wait while we load your content...