An effective strategy for creating asymmetric MOFs for chirality induction: a chiral Zr-based MOF for enantioselective epoxidation†
Abstract
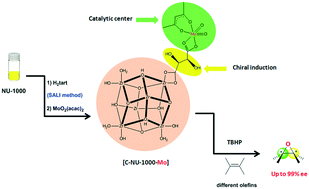
Recently the construction of chiral MOFs (CMOFs) has been very challenging and complex. For the first time, we synthesized a chiral Zr-based MOF with L-tartaric acid by solvent-assisted ligand incorporation (SALI). We show that a CMOF can be postsynthetically generated by a simple method: incorporating chiral carboxylic groups on the achiral NU-1000. The post-synthesized chiral NU-1000 was used as an asymmetric support for producing a chiral catalyst with molybdenum catalytic active centers as Lewis acid sites. Enantioselective epoxidation of various prochiral alkens to epoxids by using [C-NU-1000-Mo] is comparable to that using other asymmetric homogeneous and heterogeneous catalysts, along with high enantiomeric excess and selectivity to epoxide (up to 100%). The CMOF could be reused in the styrene oxidation after five cycles without substantial deterioration in the CMOF crystallinity or catalytic performance.
- This article is part of the themed collections: Asymmetric catalysis and 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...