Catalyzed or non-catalyzed: chemoselectivity of Ru-catalyzed acceptorless dehydrogenative coupling of alcohols and amines via metal–ligand bond cooperation and (de)aromatization†
Abstract
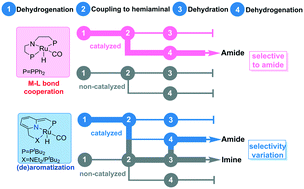
The chemoselectivities of acceptorless dehydrogenative coupling between alcohols and amines were studied using DFT methods to understand how the catalyst features and mechanisms affect the cascade catalysis. With a Macho–Ru(II) complex as a model M–L bond catalyst, three reaction stages of the catalytic cycle were investigated in detail, including (1) the dehydrogenation of alcohol to aldehyde, (2) the coupling of aldehyde with amine to hemiaminal, and (3) the dehydrogenation or dehydration of hemiaminal to amide or imine. A systematic comparison is performed between the M–L bond cooperation type Macho–Ru system and well-studied (de)aromatization systems (PNN–Ru and PNP–Ru) to elucidate the mechanistic origin of the chemoselectivity. Our study uncovers that the formation of amide is preferred over imine for the M–L bond type Macho–Ru catalyst via catalyzed pathways. Meanwhile, the selectivity between amide and imine can be varied depending on the ligand modifications, the reaction conditions, and the reactants for the (de)aromatization type pincer Ru complexes, due to the close competition between the catalyzed and the non-catalyzed pathways.


 Please wait while we load your content...
Please wait while we load your content...