Engineering of a keto acid reductase through reconstructing the substrate binding pocket to improve its activity†
Abstract
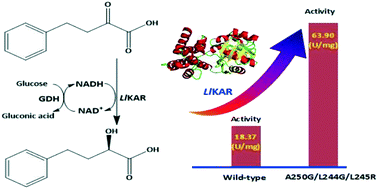
(R)-2-Hydroxy-4-phenylbutyric acid ((R)-HPBA) is a key intermediate for the synthesis of angiotensin-converting enzyme inhibitors. Asymmetric reduction of 2-oxo-4-phenylbutyric acid (OPBA) by keto acid reductases represents a promising approach for (R)-HPBA production due to its high atom economy and theoretical yield of up to 100%. However, the low activity of keto acid reductases restricts their industrial application. In this study, it is the first time that a keto acid reductase from Leuconostoc lactis (LlKAR) with superior activity was designed for the reduction of OPBA to (R)-HPBA through reconstruction of the substrate binding pocket. Several residues (Leu127, Thr129, Leu244, Leu245, and Ala250) were selected for mutagenesis. Through three-round mutagenesis, multiple positive mutants were picked out. Compared with wild-type LlKAR, the mutant L244G/A250G/L245R exhibited 3.5-fold improvement in specific activity and 6.80-fold improvement in catalytic efficiency (Kcat/Km). Using L244G/A250G/L245R coupled with Exiguobacterium sibiricum glucose dehydrogenase (GDH) for cofactor regeneration, the productivity of (R)-HPBA was up to 85.7 mM h−1 with >99% enantiomeric excess in the reaction system. The engineered L244G/A250G/L245R represents a potential and competitive biocatalyst for practical application in the synthesis of (R)-HPBA.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...