Improving the catalytic efficiency and stereoselectivity of a nitrilase from Synechocystis sp. PCC6803 by semi-rational engineering en route to chiral γ-amino acids†
Abstract
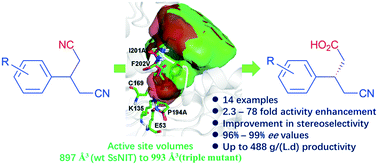
Nitrilase-catalysed desymmetrization of 3-substituted glutaronitriles to optically active 3-substituted 4-cyanobutanoic acid offers an attractive approach to access chiral β-substituted γ-amino acids, an important moiety in pharmaceuticals. In this study, we employed enzyme-substrate docking and alanine scanning to determine the key amino acid residues which have a positive effect on the activity and stereoselectivity of a nitrilase from Synechocystis sp. PCC6803 (SsNIT). Then, site-saturation mutagenesis and combinatorial mutagenesis of the positive amino acid residues (H141, P194, M197, I201 and F202) were performed, and a double mutant (P194A/F202V) and a triple mutant (P194A/I201A/F202V) with high activity and stereoselectivity were identified for desymmetrization of 3-substituted glutaronitriles to afford (S)-3-substituted-4-cyanobutanoic acids with a high space–time productivity of up to 488 g L−1 d−1. Molecular calculation suggests that the enzymatic activity improvement may be due to the enlargement of the substrate binding cavity. It is interesting that the stereoselectivity is enhanced simultaneously.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...