Acid-tolerant intermetallic cobalt–nickel silicides as noble metal-like catalysts for selective hydrogenation of phthalic anhydride to phthalide†
Abstract
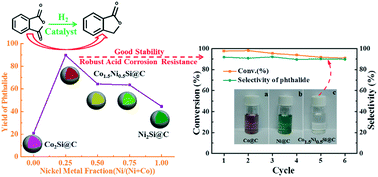
Chemoselective hydrogenation of phthalic anhydride is regarded as the most promising route for producing downstream high-performance phthalide. A series of intermetallic cobalt–nickel silicide catalysts embedded in a carbon matrix (CoxNi2−xSi@C) with acid-tolerance prepared by microwave-assisted chemical vapor deposition have been investigated in this reaction system. Activity measurements show a remarkable positive synergistic effect, forming a volcano-shaped plot over the nominal Co metal fraction for CoxNi2−xSi@C catalysts with a peak at x = 1.5, which corresponds to a 4.5-fold enhancement of the yield of phthalide over Co2Si@C and a 2.0-fold enhancement over the Ni2Si@C monometallic silicide catalysts, which can be correlated with the interaction of cobalt–nickel and metal–silicon bonds. In addition, the cobalt-rich bimetallic silicide exhibits an equal activity to the reference noble metal catalysts (Au, Pd, and Pt) and a significantly higher activity than transition metal catalysts. Moreover, the bimetallic silicide as an intermetallic compound catalyst has demonstrated robust acid corrosion resistance compared to the corresponding metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...