Mechanistic insight into the selective catalytic reduction of NO by NH3 over α-Fe2O3 (001): a density functional theory study†
Abstract
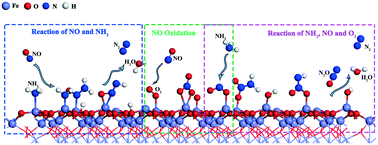
The adsorption properties and the selective catalytic reduction mechanism of NO, NH3 and O2 molecules over the α-Fe2O3 (001) surface were studied by density functional theory. The SCR reaction on the α-Fe2O3 (001) surface mainly followed the Eley–Rideal-type mechanism and three detailed reaction routes were proposed. The reaction of NO and NH3 consists of seven consecutive steps: (1) NH3 adsorption, (2) NH3 oxidation, (3) NH2NO formation, (4) NH2NO oxidation, (5) ONNH rearrangement, (2) ONNH dissociation and (7) H2O formation. –NHNO undergoes rearrangement before it dissociates to form a N2 molecule. NH3 dissociation and H2O generation are the rate-determining steps. NO oxidation has two reaction paths, NO2 generation and –NO3 formation. The reaction of NH3, NO and O2 mainly consists of three consecutive steps: (1) –NO2NH2 formation, (2) –NO2NH formation, and (3) –NO2NH dissociation. The combination of free NH2 and –NO2 forms a –NH2NO2 group, which releases an energy of 288 kJ mol−1 and plays an important role in the overall reaction as it provides energy in the following reactions. The formation and dissociation of H2O determine the SCR deNOx reaction rate and the adsorbed oxygen promotes the SCR deNOx reaction on the α-Fe2O3 (001) surface.


 Please wait while we load your content...
Please wait while we load your content...