Acceptor reactivity in glycosylation reactions
Abstract
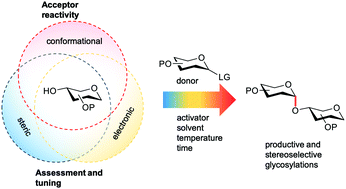
The outcome of a glycosylation reaction critically depends on the reactivity of all reaction partners involved: the donor glycoside (the electrophile), the activator (that generally provides the leaving group on the activated donor species) and the glycosyl acceptor (the nucleophile). The influence of the donor on the outcome of a glycosylation reaction is well appreciated and documented. Differences in donor reactivity have led to the development of chemoselective glycosylation reactions and the reactivity of donor glycosides has been tuned to affect stereoselective glycosylation reactions. The quantification of donor reactivity has enabled the conception of streamlined one-pot glycosylation sequences. In contrast, although it has long been known that the nature and the reactivity of the nucleophile influence the outcome of a glycosylation, the knowledge of acceptor reactivity and insight into the consequences thereof are often circumstantial or anecdotal. This review documents how the reactivity impacts the glycosylation reaction outcome both in terms of chemical yield and stereoselectivity. The effect of acceptor nucleophilicity on the reaction mechanism is described and steric, conformational and electronic influences are outlined. Quantitative and computational approaches to comprehend acceptor nucleophilicity are assessed. The increasing insight into the stereoelectronic effects governing glycoside reactivity will eventually enable the conception of effective stereoselective glycosylation methodology that can be tuned to the reaction partners at hand.


 Please wait while we load your content...
Please wait while we load your content...