Analysis of thyme essential oils using gas-phase broadband rotational spectroscopy†
Abstract
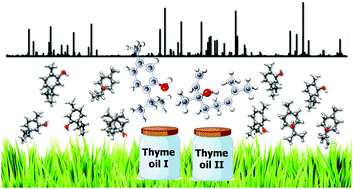
A semi-quantitative analysis of the components of two natural essential oils has been carried out using broadband rotational spectroscopy, which is inherently molecule specific. The samples under study were two thyme essential oils from Spain with different compositions: (a) with thymol as the most abundant species (thyme I) and (b) with linalool and 4-carvomenthenol being the most abundant ones (thyme II). Relative intensity measurements of selected rotational transitions were carried out to estimate the abundances of the different species present in these complex mixtures, taking into account the square of the respective dipole moment components. One strength of rotational spectroscopy is its structure sensitivity. Here, we also re-investigated the microwave spectrum of linalool and determined the accurate experimental gas-phase structures of thymol and linalool through the assignment of all 13C isotopologues of their lowest energy conformers. A characteristic splitting pattern of the rotational transitions due to internal rotation of two non-equivalent methyl groups of linalool was observed in the thyme II spectrum. Their internal rotation barriers were experimentally determined to 4.7703(96) kJ mol−1 and 9.2581(74) kJ mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...