Spectroscopic identification of the phenyltelluryl radical and its reactivity toward molecular oxygen†
Abstract
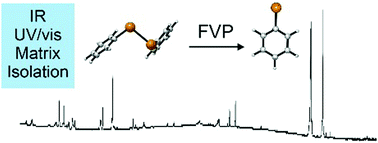
The phenyltelluryl radical was prepared by high-vacuum flash pyrolysis of diphenyl ditelluride and was chacracterized by matrix isolation IR and UV/Vis spectroscopy. After doping the matrix with molecular oxygen and allowing bimolecular reactions, the hitherto unkown phenyltelluro peroxy radical formed and was identified via IR spectroscopy. Irradiation with light at λ = 436 nm leads to isomerization to the thermodynamically more stable novel phenyltelluroyl radical. All experimental findings agree well with density functional theory (UB3LYP/Def2QZVPP and UM06-2X/Def2QZVPP) computations.


 Please wait while we load your content...
Please wait while we load your content...