Conformational isomerizations triggered by vibrational excitation of second stretching overtones†
Abstract
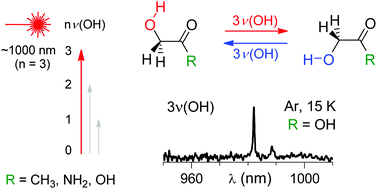
Vibrational excitation using frequency-tunable IR laser light has been developed as a powerful tool for selective manipulation of molecular conformations. In this methodology, vibrational excitation has been typically applied to the first stretching overtones (∼80 kJ mol−1) but also to the fundamental modes (∼40 kJ mol−1). Here, we demonstrate that selective conformational isomerizations are also achieved using excitation to second stretching overtones (∼120 kJ mol−1). The extremely weak absorptions of the second stretching overtones of molecules isolated in low-temperature matrices were measured for the first time; here using three prototype molecules: hydroxyacetone (HA), glycolic acid (GAc) and glycolamide (GAm). Benchmarking of computed anharmonic IR spectra showed that the B3LYP/SNSD method provides the best agreement with experimental frequencies of the ν(OH), 2ν(OH) and 3ν(OH) modes for the studied molecules in argon matrices. Selective irradiation at the 3ν(OH) frequencies (9850–10 500 cm−1) of HA, GAc and GAm monomers in argon matrices at 15 K successfully triggers their conformational isomerization. These results open the door to extend control over conformations separated by higher barriers and to induce other transformations not energetically accessible by excitation to the fundamental or first stretching overtone modes.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...