Transition from exohedral to endohedral geometries of anionic and neutral B4Sin (n = 4–15) clusters: quantum chemical calculations†
Abstract
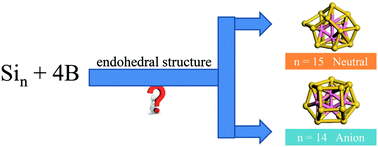
The growth patterns of anionic and neutral B4Sin (n = 4–15) clusters are investigated by using density functional theory (DFT) calculations combined with particle swarm optimization (CALYPSO) software. The geometries of anionic and neutral B4Sin clusters transform from exohedral to endohedral structures with the increasing cluster sizes. The B4Si14− anion size is critical for forming B4-endohedral structures for anionic clusters, while the B4Si15 neutral size is the threshold size for forming B4-endohedral structures for neutral clusters. Both anionic and neutral B4Sin (n = 4–15) clusters are primarily dominated by prism-based or bipyramid-based geometries. The global minima of anionic and neutral B4Sin clusters adopt different geometrical structures, except for anionic and neutral B4Si10. The binding energies, second-order energy differences, and incremental binding energies of B4Sin− clusters show an odd–even alternation with the growing number of Si atoms. The B atoms in B4Sin−/0 exhibit B–B single bonding and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bonding properties. The B atoms are found to carry more negative charges due to charge transfer from Sin frameworks to B atoms. Interestingly, the B4Si4− anion adopts a C2h symmetric bicapped tetragonal bipyramid with σ plus π double bonding characters and has the highest relative stability among the anionic clusters. The bonding interactions in B4Si4− are in the order of B–B > B–Si > Si–Si.
B double bonding properties. The B atoms are found to carry more negative charges due to charge transfer from Sin frameworks to B atoms. Interestingly, the B4Si4− anion adopts a C2h symmetric bicapped tetragonal bipyramid with σ plus π double bonding characters and has the highest relative stability among the anionic clusters. The bonding interactions in B4Si4− are in the order of B–B > B–Si > Si–Si.


 Please wait while we load your content...
Please wait while we load your content...