Fluorine conformational effects characterized by energy decomposition analysis†
Abstract
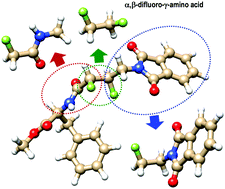
Electrostatic and stereoelectronic effects associated with fluorine atoms can be exploited as conformational tools for the design of shape-controlled functional molecules. To gain further insight into the nature and strength of these effects, we use the Interacting Quantum Atoms (IQA) method augmented with the semiclassical pairwise dispersion potential to decompose the conformational energies of fluoro-substituted molecules into fragment-based energy contributions, which include deformation/distortion terms and the electrostatic, exchange–correlation and dispersion interactions. The studied molecules comprise various F–CH2–CH2–X and F–CH2–CO–X systems, as well as selected conformers of an α,β-difluoro-γ-amino-acid derivative that is potentially useful for the design of shape-controlled bioactive amino acids and peptides. We identify the most relevant exchange–correlation and/or electrostatic interaction terms contributing to the stability of the various conformers, and we show that IQA can be used to assess the gauche/anti or trans/cis preferences in molecules with two or more rotatable bonds as well as to study the roles played by other concomitant effects (e.g., CH/OH/NH⋯F contacts). For the α,β-difluoro-γ-amino acid derivatives, our theoretical analysis indicates that the gauche/anti and trans/cis effects associated with fluorine bonds can be significantly attenuated by other specific intra-molecular contacts.


 Please wait while we load your content...
Please wait while we load your content...