The synergistic effects of Cu clusters and In2O3 on ethanol synthesis from acetic acid hydrogenation
Abstract
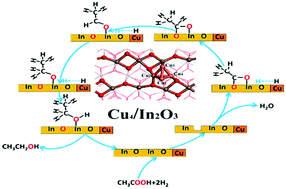
The development of high efficiency catalysts for acetic acid hydrogenation to ethanol could ameliorate the petroleum crisis and acetic acid overproduction. Cu and In2O3 catalysts both show catalytic activity for acetic acid hydrogenation. However, monometallic Cu catalysts are less active in the dissociative adsorption of acetic acid through C–O bond breaking, while the H2 adsorption and dissociation ability of In2O3 is weak. In this work, Cu4/In2O3 is designed to enhance the dissociation of both acetic acid and H2. The detailed mechanism of acetic acid hydrogenation to ethanol on Cu4/In2O3 is explored using periodic density functional theory (DFT). The results show that the H2 adsorption and dissociation are enhanced by the Cu cluster, while the H atom spillover from Cu to In2O3 is favorable on the Cu4/In2O3(110) surface. Additionally, a synergistic effect exists between the Cu cluster and In2O3 surface: H2 adsorbs on the Cu cluster and the dissociated H atoms react with acetic acid activated by the In2O3 oxygen vacancy. Finally, compared with a Cu2In(100) surface, the Cu4/In2O3(110) surface possesses higher catalytic activity owing to the reduced energy barriers of acetic acid dissociation and hydrogenation of the intermediates (CH3COO*, CH3CHO*, and CH3CH2O*). The Cu4/In2O3 catalyst proposed in this work can provide promising guidance for related catalyst design.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...