Allosteric modulation of the sarcoplasmic reticulum Ca2+ ATPase by thapsigargin via decoupling of functional motions†
Abstract
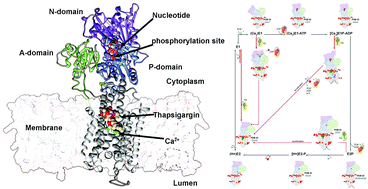
The sarcoplasmic reticulum Ca2+-ATPase (SERCA) is a widely studied member of the large family of phosphorylation(P)-type ATPase membrane transporters. Ligands and nucleotide binding naturally modulate the conformational space of P-type ATPases through allosteric inter-domain communications. Whereas many inhibitory ATPase ligands act by directly blocking substrate uptake or release, SERCA is a target for thapsigargin (TG), a plant-derived natural product that allosterically inhibits the transport cycle. While thapsigargin's inhibitory effects on SERCA have been widely studied experimentally, the molecular mechanisms underlying these remain incompletely understood. Here, we apply modelling and molecular simulations to probe the effects of TG binding to the major functional states along SERCA's reaction cycle. Our results provide insight into the atomic-level details of the conformational changes induced by TG binding to SERCA, and suggest mechanisms for its effect. Since other P-type ATPases share closely related reaction cycles, our data suggests that similar modulators might exist for these.


 Please wait while we load your content...
Please wait while we load your content...