Molecular and electronic structure of an azidocobalt(iii) complex derived from X-ray crystallography, linear spectroscopy and quantum chemical calculations†
Abstract
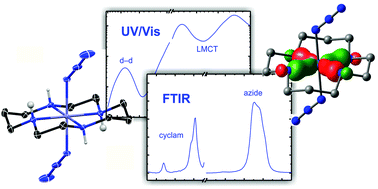
The photochemistry of transition-metal azides is remarkably complex and can involve multiple competing pathways leading to different fragmentation patterns. Therefore, an in-depth study of such rich photochemistry requires a thorough prior understanding of the molecular and electronic structures of these complexes. To this end, stationary (i.e. linear) spectroscopies in the ultraviolet-to-visible (UV/vis) and the mid-infrared (MIR) spectral regions are most often employed. Here, we investigate the electronic and vibrational spectroscopies of the cationic diazidocobalt(III) complex, trans-[Co(cyclam)(N3)2]+, in liquid dimethyl sulfoxide (DMSO) solution and interpret the experimental data in terms of detailed quantum chemical calculations. The X-ray crystallography reveals a Ci-symmetric molecular structure of the complex whereas in liquid solution, evidence for symmetry breakage with loss of the inversion center of the ligand sphere is found from both, the UV/vis and MIR-data. This interpretation is fully corroborated by a stereochemical and conformational analysis of the complex using ab initio calculations involving nuclear degrees of freedom of both, the equatorial cyclam ancillary ligand and the two axial azido ligands.


 Please wait while we load your content...
Please wait while we load your content...