Electrochemical CO2 reduction on Cu and Au electrodes studied using in situ sum frequency generation spectroscopy†
Abstract
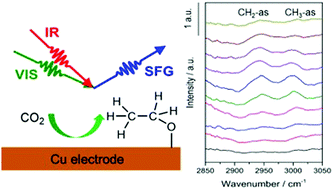
As an important pathway for energy storage and a key reaction in the carbon cycle, the CO2 electrochemical reduction reaction has recently gained significant interest. A variety of catalysts have been used to approach this topic experimentally and theoretically; however, the molecular level insight into the reaction mechanism is lacking due to the complexity of the surface processes and the challenges in probing the intermediate species. In this study, CO2 reduction reactions on polycrystalline Cu and Au electrodes were investigated in 0.1 M CO2-saturated NaHCO3 solution. In situ sum frequency generation (SFG) spectroscopy has been adopted to access the intermediates and products on the metal electrodes. On the Au electrode, only linearly adsorbed CO could be detected, and the reduction produced no hydrocarbon species. On the Cu electrode, C–H stretching vibrations corresponding to surface-adsorbed ethoxy species were observed, but no CO vibrations can be detected with SFG. The results revealed that the CO randomly adsorbed on the Cu surface, and the multiple orientations of the adsorbed species may be the reason for the formation of C–C bonding. These results demonstrate direct molecular level evidence for different reaction pathways on the Cu and Au electrodes.


 Please wait while we load your content...
Please wait while we load your content...