Atmospheric oxidation mechanism and kinetics of 2-bromo-4,6-dinitroaniline by OH radicals – a theoretical study†
Abstract
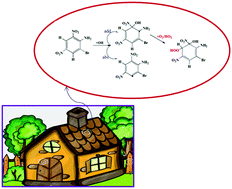
2-Bromo-4,6-dinitroaniline (BNA) is identified as a domestic-dust pollutant in urban environments, with deleterious atmospheric effects. In the present work, we studied the reaction pathways and kinetics for BNA oxidation by the OH radical using quantum-chemical methods and canonical-variational transition-state theory with small-curvature tunneling correction (CVT/SCT). OH-radial-mediated BNA oxidation was studied by considering OH addition to carbon atoms (C1 to C6) of BNA and H-atom abstraction at the –NH2 group and carbon atoms (C3 and C5) of BNA by OH radicals. It is observed that an OH-addition reaction is energetically more favorable. In addition, the rate constant was calculated for the favorable initial OH-addition reactions over the temperature range of 278 to 1000 K. The subsequent reactions for the favorable BNA-OH adduct intermediate with O2, HO2 and NO radicals are studied. We have identified the following possible end products from this BNA-oxidation reaction: (i) 2-amino-3-bromo-6-hydroperoxy-5-methyl-1-nitro-cyclohexa-2,4 dienol, (ii) 2-amino-1-bromo-6-hydroperoxy-5-methyl-3-nitro-cyclohexa-2,4-dienol, (iii) 2-amino-1-bromo-6-hydroperoxy-5-methyl-3-nitro-cyclohexa-2,4-dienol, (iv) 3-amino-4-bromo-4-hydroperoxy-8-methyl-2-nitro-6,7-dioxa-bicyclo oct-2-en-8-ol, (v) 2-amino-1-bromo-6-hydroperoxy-5-methyl-3-nitro-cyclohexa-2,4-dienol, and (vi) 3-amino-2-bromo-8-methyl-4-nitro-6,7-dioxa-bicyclo oct-3-ene-2,8-diol.


 Please wait while we load your content...
Please wait while we load your content...