Detailed mechanism and kinetics of the reaction of Criegee intermediate CH2OO with HCOOH investigated via infrared identification of conformers of hydroperoxymethyl formate and formic acid anhydride†
Abstract
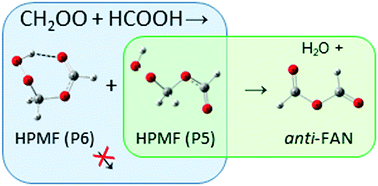
The reaction of Criegee intermediate CH2OO with HCOOH is important in atmospheric chemistry, but its mechanism and kinetics are little investigated. We recorded time-resolved infrared absorption spectra of transient species produced upon irradiation at 308 nm of a flowing mixture of CH2I2/O2/N2/HCOOH at 298 K. Bands of CH2OO were observed initially upon irradiation; their decrease was accompanied with the appearance of several bands near 887, 925, 1052, 1115, 1170, 1342, 1391, and 1760 cm−1, assigned to the absorption of hydroperoxymethyl formate [HC(O)OCH2OOH, HPMF], that decreased in intensity at a later period with the appearance of absorption bands of the anti-conformer of formic acid anhydride [anti-(HCO)2O, FAN] near 998, 1101, 1767, and 1821 cm−1. The main contributions of the infrared absorption of HPMF are from an open-form conformer, but small contributions from the intramolecular hydrogen-bonded conformer that absorbs near 1070, 1170, and 1732 cm−1 were identified. Observed infrared spectra of both conformers of HPMF and anti-FAN agree satisfactorily with the anharmonic vibrational wavenumbers and IR intensities predicted with the B3LYP/aug-cc-pVTZ method. We derived a rate coefficient for CH2OO + HCOOH to be k = (1.4 ± 0.3) × 10−10 cm3 molecule−1 s−1 from formation of HPMF. We found also that anti-FAN was produced mainly from the open-form conformer with rate coefficient k = (1460 ± 30) s−1; the intramolecular hydrogen-bonded conformer of HPMF is stable.


 Please wait while we load your content...
Please wait while we load your content...