Anion binding to mutants of the Schiff base counterion in heliorhodopsin 48C12†
Abstract
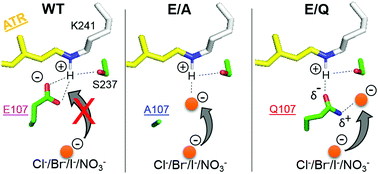
Heliorhodopsin (HeR), a recently discovered new rhodopsin family, contains a single counterion, E107, which is specific to HeR 48C12. In this paper, we examined possible anion binding into the wild-type (WT) and E107 mutants of HeR 48C12. We prepared E107A, E107Q, and E107D, and chloride binding was tested by measuring absorption spectra using UV-visible spectroscopy under various anionic conditions. The experimental results clearly showed no anion binding to WT and E107D, where E107 and D107 acted as the Schiff base counterion, respectively. On the other hand, anion binding was observed to the Schiff base region in E107A and E107Q. In the case of E107A, λmax was 553, 559, 565, and 549 nm for Cl−, Br−, I−, and NO3−, respectively. Similar halide-size dependence on the absorption spectra of the chromophore in solution strongly suggests that the anion acted as the direct hydrogen-bonding acceptor of the protonated Schiff base in E107A. In the case of E107Q, λmax was 577, 578, 579, and 581 nm for Cl−, Br−, I−, and NO3−, respectively. Based on the small halide dependence, we interpreted the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the Q107 side chain as the hydrogen-bonding acceptor. Moreover, the anion stabilized the protonated state without a direct hydrogen bond with the Schiff base. Structures around the Schiff base region are discussed for the WT and E107 mutants of HeR 48C12.
O group of the Q107 side chain as the hydrogen-bonding acceptor. Moreover, the anion stabilized the protonated state without a direct hydrogen bond with the Schiff base. Structures around the Schiff base region are discussed for the WT and E107 mutants of HeR 48C12.


 Please wait while we load your content...
Please wait while we load your content...