Stabilizing lead halide perovskites with quaternary ammonium cations: the case of tetramethylammonium lead iodide†
Abstract
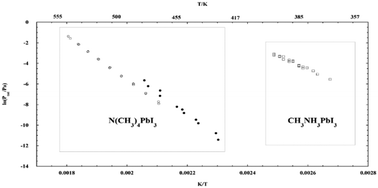
Organoammonium lead halide perovskites, especially methylammonium lead iodide CH3NH3PbI3, are promising photovoltaic materials, but they are far from commercial applications due in particular to their thermal instability and moisture sensitivity. Here, we present a multitechnique study aimed at investigating the kinetic and thermodynamic stability of the simplest quaternary ammonium lead iodide, tetramethylammonium lead iodide N(CH3)4PbI3. The kinetics of thermal decomposition was studied by X-ray powder diffraction of samples treated in air at different temperatures combined with Rietveld quantitative phase analysis, and by the isoconversional analysis of differential thermal analysis measurements. Evidence for first order kinetics was obtained, with an activation energy of 280–290 kJ mol−1, suggesting that the breaking of the C–N bond is the rate determining step. The composition of the gas phase released under heating was investigated by Knudsen Effusion Mass Spectrometry, giving evidence for the occurrence of the process N(CH3)4PbI3(s) = PbI2(s) + N(CH3)3(g) + CH3I(g), consistent with the kinetic results. Decomposition pressures and thermodynamic properties were derived by Knudsen effusion mass loss experiments, obtaining values of 391.5 ± 2.0 kJ mol−1 and −577.4 ± 4.0 kJ mol−1 for the decomposition and formation enthalpies at 298 K, respectively. The reactivity towards water of N(CH3)4PbI3 was checked by XRD after total and prolonged immersion in water at room temperature. Overall, N(CH3)4PbI3 was found to be thermally much more stable than CH3NH3PbI3, both kinetically and thermodynamically, and much less prone to water-induced degradation, suggesting that the use of a quaternary ammonium cation may be an effective strategy in order to produce more stable materials.


 Please wait while we load your content...
Please wait while we load your content...