QM/MM simulations of organic phosphorus adsorption at the diaspore–water interface†
Abstract
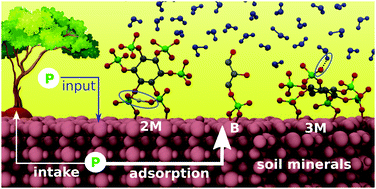
Phosphorus (P) immobilization and thus its availability for plants are mainly affected by the strong interaction of phosphates with soil components especially soil mineral surfaces. The related reactions have been studied extensively via sorption experiments especially by carrying out adsorption of ortho-phosphates onto Fe-oxide surfaces. But a molecular-level understanding of the P-binding mechanisms at the mineral–water interface is still lacking, especially for forest eco-systems. Therefore, the current contribution provides an investigation of the molecular binding mechanisms for two abundant phosphates in forest soils, inositol hexaphosphate (IHP) and glycerolphosphate (GP), at the diaspore mineral surface. Here a hybrid electrostatic embedding quantum mechanics/molecular mechanics (QM/MM) based molecular dynamics simulation has been applied to explore the diaspore–IHP/GP–water interactions. The results provide evidence for the formation of different P-diaspore binding motifs involving monodentate (M) and bidentate (B) for GP and two (2M) as well as three (3M) monodentates for IHP. The interaction energy results indicated the abundance of the GP B motif compared to the M one. The IHP 3M motif has a higher total interaction energy compared to its 2M motif, but exhibits a lower interaction energy per bond. Compared to GP, IHP exhibited stronger interaction with the surface as well as with water. Water was found to play an important role in controlling these diaspore–IHP/GP–water interactions. The interfacial water molecules form moderately strong H-bonds (HBs) with GP and IHP as well as with the diaspore surface. For all the diaspore–IHP/GP–water complexes, the interaction of water with the diaspore exceeds that with the studied phosphates. Furthermore, some water molecules form covalent bonds with diaspore Al atoms while others dissociate at the surface to protons and hydroxyl groups leading to proton transfer processes. Finally, the current results confirm the previous experimental conclusions indicating the importance of the number of phosphate groups, HBs, and proton transfers in controlling the P-binding at soil mineral surfaces.


 Please wait while we load your content...
Please wait while we load your content...