How calcium ion binding induces the conformational transition of the calmodulin N-terminal domain—an atomic level characterization†
Abstract
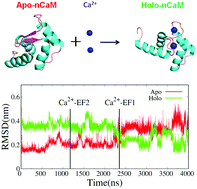
Allostery plays important roles in the regulation of many biological processes, such as signal transduction and transcriptional regulation. Although great advances have been achieved in understanding the allosteric mechanism through experimental and theoretical investigations, the details of the allosteric process are still not clear. Here, using the N-terminal domain of calmodulin (nCaM) as the model protein, we reported the atomic level characterization of the allosteric process induced by Ca2+ binding through extensive and unbiased molecular dynamics simulations. In two trajectories, it was found that Ca2+ first binds to EF-hand 2 and then induces the conformational transformation of nCaM from the Apo to Holo state assisted by second Ca2+ binding to EF-hand 1 completely. The binding order was consistent with a recent experimental result. The simulations also indicated that the two EF-hands changed conformations synergistically and the EF-hand 2 showed an earlier and more gradual conformational transition. Meanwhile, the allosteric process of nCaM triggered by Ca2+ binding might be completed within hundreds of nanoseconds in a two-state-like manner. This was validated by biased simulations, in which the Ca2+ ions were restrained near the binding sites. This work provides the molecular details of the conformational transition of nCaM triggered by Ca2+ binding.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...