Rationalizing the diversity of amide–amide H-bonding in peptides using the natural bond orbital method†
Abstract
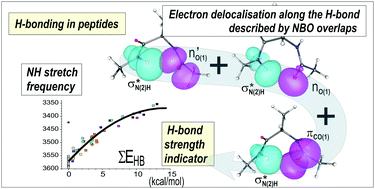
Natural bond orbital (NBO) analysis of electron delocalization in a series of capped isolated peptides is used to diagnose amide–amide H-bonding and backbone-induced hyperconjugative interactions, and to rationalize their spectral effects. The sum of the stabilization energies corresponding to the interactions between NBOs that are involved in the H-bonding is demonstrated as an insightful indicator for the H-bond strength. It is then used to decouple the effect of the H-bond distance from that, intrinsic, of the donor/acceptor relative orientation, i.e., the geometrical approach. The diversity of the approaches given by the series of peptides studied enables us to illustrate the crucial importance of the approach when the acceptor is a carbonyl group, and emphasizes that efficient approaches can be achieved despite not matching the usual picture of a proton donor directly facing a lone pair of the proton acceptor, i.e., that encountered in intermolecular H-bonds. The study also illustrates the role of backbone flexibility, partly controlled by backbone–amide hyperconjugative interactions, in influencing the equilibrium structures, in particular by frustrating or enhancing the HB for a given geometrical approach. Finally, the presently used NBO-based HB strength indicator enables a fair prediction of the frequency of the proton donor amide NH stretching mode, but this simple picture is blurred by ubiquitous hyperconjugative effects between the backbone and amide groups, whose magnitude can be comparable to that of the weakest H-bonds.


 Please wait while we load your content...
Please wait while we load your content...