Sulfur adsorption on coinage metal(100) surfaces: propensity for metal–sulfur complex formation relative to (111) surfaces
Abstract
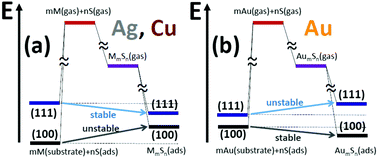
Experimental data from low-temperature Scanning Tunneling Microscopy (LTSTM) studies on coinage metal surfaces with very low coverages of S is providing new insights into metal–S interactions. A previous LTSTM study for Cu(100), and a new analysis reported here for Ag(100), both indicate no metal–sulfur complex formation, but an Au4S5 complex was observed previously on Au(100). In marked contrast, various complexes have been proposed and/or observed on Ag(111) and Cu(111), but not on Au(111). Also, exposure to trace amounts of S appears to enhance mass transport far more dramatically on (111) than on (100) surfaces for Cu and Ag, a feature tied to the propensity for complex formation. Motivated by these observations, we present a comprehensive assessment at the level of DFT to assess the existence and stability of complexes on (100) surfaces, and compare results with previous analyses for (111) surfaces. Consistent with experiment, our DFT analysis finds no stable complexes on Ag(100) and Cu(100), but several exist for Au(100). In addition, we systematically relate stability for adsorbed and gas-phase species within the framework of Hess's law. We thereby provide key insight into the various energetic contributions to stability which in turn elucidates the difference in behavior between (100) and (111) surfaces.


 Please wait while we load your content...
Please wait while we load your content...