Ionic liquid based battery electrolytes using lithium and sodium pseudo-delocalized pyridinium anion salts†
Abstract
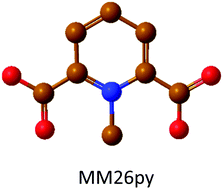
The electrolyte salt plays an important role for the overall performance and safety of lithium- and sodium-ion batteries (LIBs and SIBs, respectively). Here, two new lithium and sodium pseudo-delocalized pyridinium anion based salts were used to prepare ionic liquid (IL) based electrolytes. The Li and Na salts of the 1-methylpyridinum 2,6-dicarboxylate anion (MM26py) were synthesized and dissolved in an IL matrix (Pyr14TFSI) – hence creating mixed anion electrolytes. The obtained electrolytes are stable up to 150 and 200 °C and show ion conductivities of 2.8 and 3.2 mS cm−1 at room temperature, for the LIB and SIB electrolytes, respectively. A competitive effect between the MM26py and the TFSI anions to coordinate the alkali metal cations is observed. Finally, the electrochemical stability windows of 2.3 and 2.5 V, respectively, confirm that these electrolytes can be used practically in medium-voltage LIBs and SIBs.


 Please wait while we load your content...
Please wait while we load your content...