Exploring the mechanism of hypochlorous acid decomposition in aqueous solutions†
Abstract
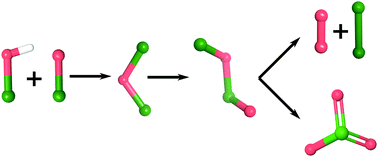
Hypochlorous acid is an intermediate in important industrial processes such as the production of chlorate but is also used for water treatment and disinfection. In aqueous solutions hypochlorous acid may decompose into oxygen or chlorate. Using density functional theory (DFT) modelling we have for the first time established detailed mechanisms for the respective decomposition pathways. Our calculations indicate, that both oxygen and chlorate formation proceed through an identical set of intermediates. At neutral pH the reaction is initiated by a fast equilibrium between HOCl, OCl−, Cl2O and Cl3O2−. The subsequent abstraction of Cl− to form Cl2O2 is rate determining for chlorate formation while it is the decomposition of Cl2O2 in the case of oxygen formation. Under alkaline conditions, OCl− decomposition to chlorate proceeds through chlorite. This reaction path is significantly less active. The highest rate for chlorate or oxygen formation is found at pH 7.1. These results highlight the need to consider a complex mixture of different Cl species when addressing the chemistry of hypochlorous acid containing solutions.


 Please wait while we load your content...
Please wait while we load your content...