S–H rotamerization via tunneling in a thiol form of thioacetamide†
Abstract
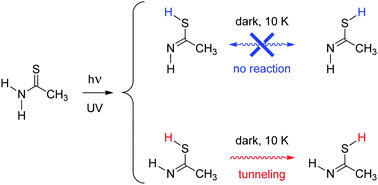
Rotamerization of a hydroxyl (O–H) group by tunneling is well-known and has been extensively studied. On the other hand, similar tunneling processes for the thiol (S–H) group have not been reported yet. In this work, the imino-thiol forms of thioacetamide were studied in cryogenic matrices (Ar, Xe) after UV-irradiation of the common amino-thione form of the compound. Four different imino-thiol forms were generated, corresponding to the cis or trans thiol (C/T) conformers of the two imino isomers (syn and anti; s/a). Noteworthy, the syn-cis (sC) imino-thiol form was found to convert spontaneously to the syn-trans (sT) form (with a half-life of 80 min), in a process whose reaction rate is independent of the temperature (i.e., at 11 or 20 K). Such conformational transformation represents the first experimental observation of an S–H rotamerization occurring by tunneling. Computations based on the Wentzel–Kramers–Brillouin formalism predict a tunneling half-life for the S–H rotamerization of syn-imino sC to sT on the time scale of minutes, in agreement with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...