Chalcogen bond and internal dynamics of the 2,2,4,4-tetrafluoro-1,3-dithietane⋯water complex†
Abstract
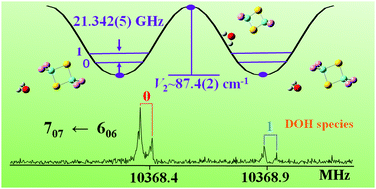
The rotational spectrum of the 2,2,4,4-tetrafluoro-1,3-dithietane⋯water complex has been investigated by high resolution rotational spectroscopy. Experimental evidence and quantum theoretical analyses revealed that the two moieties are linked together through a dominant S⋯O chalcogen bond. Two secondary F⋯O interactions contribute to the stability of the complex. The rotational transitions of four isotopologues are split into two component lines due to the internal rotation of the water moiety around its C2 axis. In the HDO isotopologue, a small μc dipole moment component is generated which inverts upon internal rotation of water, allowing the experimental determination of the tunneling splitting (21.46(5) GHz). Such splitting can be reproduced with a one-dimensional flexible model when the barrier to internal rotation of water is 87.4(2) cm−1.


 Please wait while we load your content...
Please wait while we load your content...