A coarse-grained model of ionic liquid crystals: the effect of stoichiometry on the stability of the ionic nematic phase†
Abstract
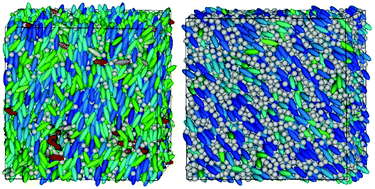
We have investigated, by means of molecular dynamics simulations, the phase behaviour of mixtures of charged ellipsoidal Gay-Berne (GB) particles and spherical Lennard-Jones (LJ) particles, as a coarse-grained model of ionic liquid crystals (ILCs). The anisotropic GB particles represent cations usually found in ILCs, for example, pyridinium or bipyridinium salts, while the spherical LJ particles are taken as a model of anions like common halides, hexafluorophosphate and tetrafluoroborate. Here we have focused our attention on the effect of the stoichiometry of the system (that is, the GB : LJ ratio n : m in the salt formula [GB]n[LJ]m) on the stability and thermal range of the ionic liquid crystal phases formed, with special attention to the ionic nematic phase. To isolate the stoichiometry effect, a comparison of four different systems with GB : LJ ratios of 1 : 3, 1 : 2, 1 : 1 and 2 : 1 is made by keeping the packing fraction and the charge of the minor component fixed. Our results suggest a way to improve the stability of the ionic nematic phase by enhancing the anisotropic van der Waals interaction compared to the Coulomb interaction, and by increasing the proportion of anisotropic particles in the mixture.
- This article is part of the themed collection: PCCP Editor’s Choice, 2020


 Please wait while we load your content...
Please wait while we load your content...