Theoretical insight into the single-atom catalytic mechanism of CeO2-supported Ag catalysts in CO oxidation†
Abstract
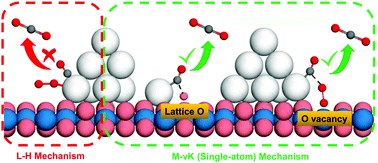
Revealing the accurate active center structure and the functional mechanism of CeO2-supported Ag catalysts during catalysis is extremely important for their accurate synthesis. In this work, a series of AgnCeO2 (n = 1, 2, 3, 4 and 10) model catalysts was constructed, and a DFT investigation of the reaction mechanism of CO oxidation, as a probe reaction on those catalysts, was carried out. It was found that the entire catalytic reaction was completed coordinately by Ag, lattice O and O vacancies, which could be considered as the active centers. Noticeably, the mobility of Ag atoms played an important role in the reaction process, leading to the observation of a single-atom catalytic mechanism, wherein a series of single Ag atomic species was formed during the reaction, which was beneficial to CO oxidation. With the completion of some elementary reactions, the single Ag formed during the migration of CO–Ag could return to the Ag cluster again. As expected, the single-AgCeO2 catalyst exhibited extremely high activity due to the absence of the binding effect of Ag–Ag. Nevertheless, the AgnCeO2 (n > 1) catalysts showed similar catalytic activity, which was slightly worse than that of single AgCeO2, indicating that the size effect of the Ag cluster was not obvious. These results provide the theoretical basis for further understanding the functional mechanism of the AgnCeO2 catalyst and are helpful for designing various catalysts with tailored functionalities.


 Please wait while we load your content...
Please wait while we load your content...