A comparative study on modeling of the ferromagnetic and paramagnetic states of uranium hydride using a DFT+U method†
Abstract
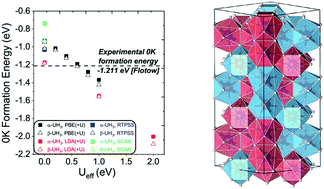
Uranium hydride is a promising material for stationary hydrogen storage in fusion reactors. In this work, various material properties of uranium hydride in both ferromagnetic (FM) and paramagnetic (PM) states are calculated to determine the optimal first-principles calculation method. For the treatment of strongly correlated f-electrons, the PBE functional with a Hubbard U parameter of 0.6 eV is selected as the optimal method and provides accurate formation energies and reasonable structural properties of the FM state. Using this method, we test four model spin configurations to approximately simulate the PM state: FM, antiferromagnetic (AFM), special quasi-random structure (SQS) and nonmagnetic (NM) configurations. The FM and AFM configurations provide formation energy and lattice constants comparable to those of the SQS configuration, which is used as the reference PM state. In addition, the experimental results on thermal expansion and the bulk modulus in the PM states are well reproduced with the FM, AFM and SQS configurations. These results demonstrate that PBE+U with FM, AFM and SQS configurations can approximately simulate the PM states, although there are some properties that can only be qualitatively reproduced by DFT calculations, such as the magnetic transition. This study enables the design of multiscale modeling for uranium hydride while maintaining simultaneous efficiency and accuracy.


 Please wait while we load your content...
Please wait while we load your content...