Theoretical exploration of 2,2′-bipyridines as electro-active compounds in flow batteries†
Abstract
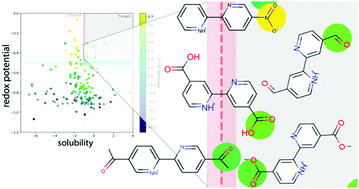
Compounds from the 2,2′-bipyridine molecular family were investigated for use as redox-active materials in organic flow batteries. For 156 2,2′-bipyridine derivatives reported in the academic literature, we calculated the redox potential, the pKa for the second deprotonation reaction, and the solubility in aqueous solutions. Using experimental data on a small subset of derivatives, we were able to calibrate our calculations. We find that functionalization with electron-withdrawing groups leads to an increase of the redox potential and to an increase of the molecular acidity (as expressed in a reduction of the pKa value for the second deprotonation step). Furthermore, calculations of solubility in water indicate that some of the studied derivatives have adequate solubility for flow battery applications. Based on an analysis of the phyisco-chemical properties of the 156 studied compounds, we down-select five molecules with carbonyl- and nitro-based functional groups, whose parameters are especially promising for potential applications as negative redox-active materials in organic flow batteries.


 Please wait while we load your content...
Please wait while we load your content...