Formation of α-helical and β-sheet structures in membrane-bound human IAPP monomer and the resulting membrane deformation†
Abstract
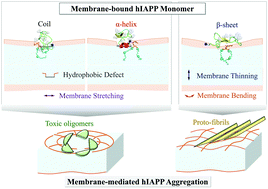
The amyloid formation of human islet amyloid polypeptide (hIAPP)—an intrinsically disordered peptide, is associated with type II diabetes. Cellular membranes, especially those composed of negatively-charged lipids, accelerate the hIAPP amyloid fibrillation, and their integrity is disrupted during the aggregation process, leading to cell apoptosis. However, the underlying molecular mechanism is not well understood. Herein, we investigated the conformational dynamics during the interactions of hIAPP monomer with POPG membrane bilayer, by carrying out μs-long all-atom molecular dynamics simulations. Starting from the metastable coiled conformations in water, hIAPP monomers tend to adopt transient α-helical and β-sheet structures when adsorbing to the membrane surface. The amphiphilic N-terminal region further inserts into the membrane interior and is located at the lipid head–tail interface, mainly in turn and α-helical structures. In contrast, the β-hairpin structures reside on the membrane surface without insertion, and expand laterally with the hydrophobic residues exposed to the solvent. Moreover, the adsorption and insertion of hIAPP monomers induce two distinct local membrane deformations: (1) the hIAPP adsorption on the membrane surface mainly causes membrane bending; (2) the insertion of both turns and α-helices synchronizes with the formation of hydrophobic defects on the POPG membrane, leading to stronger membrane stretching and a longer coherence length of membrane thinning. Based on the structural and dynamical results, we propose that β-hairpin structures may be a precursor for the fibrillation on the membrane surface due to the flat geometry and hydrophobic regions exposed to solvent, while N-terminal amphiphilic α-helices would facilitate hIAPP assembling into toxic oligomers inside the membrane.


 Please wait while we load your content...
Please wait while we load your content...