Magnetic descriptors of hydrogen bonds in malonaldehyde and its derivatives
Abstract
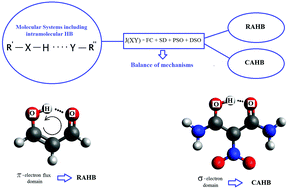
The nature of the hydrogen bond, HB, as such is still unknown, though a few of its most fundamental features has been uncovered during the last few decades. At the moment, it is possible to obtain reliable results for only a few of its broadest properties, like magnetic properties. They could give new insights into the physics underlying the strength and features of HBs. In this article we analyze the electronic origin of the NMR spectroscopic parameters of malonaldehyde, MA, and some substituted MAs. These substituted MAs are such that the H-bonds are assisted by one of two phenomena: resonance, RAHB, or charge, CAHB. We have studied the dependences of these parameters on two of the main factors which contribute the most to both phenomena, the geometrical and electronic factors, and found out how they can be used to characterize RAHB or CAHB by means of reliable theoretical calculations. We show that in the set of compounds analyzed here (i) the shielding of the proton of the H-bond can be used as a measure of the strength of the HB and (ii) the relation between the contact and non-contact mechanisms of J-couplings between donor and acceptor atoms is a reliable descriptor of whether the H-bond is resonance assisted or charge assisted.


 Please wait while we load your content...
Please wait while we load your content...