Anti-parallel dimer and tetramer formation of cyclic and open structure tertiary amides, N-methyl-2-pyrrolidone and N,N-dimethylacetamide, in solution of a non-polar solvent, benzene†
Abstract
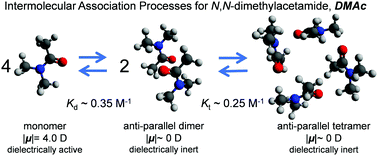
We examined the dielectric (DE) and nuclear magnetic resonance (NMR) spectroscopic behaviour of N-methyl-2-pyrrolidone (NMP) and N,N-dimethylacetamide (DMAc), which are tertiary amide compounds that bear five-membered cyclic and open structures, respectively. They were examined to investigate the formation of anti-parallel dimers and tetramers in a dipole configuration in the pure liquid state and in benzene solution over a wide concentration range at 25 °C. Because the Kirkwood correlation factors of electric dipoles considerably decreased with increasing concentrations in both the NMP and DMAc systems and because the second DE relaxation modes were clearly observed to exhibit relaxation times that are longer than those of the first modes assigned to the rotational relaxation of monomeric molecules, anti-parallel dimers are formed due to dipole–dipole interactions with increasing concentrations. Assuming a chemical process between the monomers (MONs) and the anti-parallel dimer (DIM), 2MON ⇄ DIM, the equilibrium constants for the anti-parallel dimer formation were determined to be KDEd ∼ 1.2 M−1 for NMP and 0.35 M−1 for DMAc, based on the first DE relaxation strength. The fact that the evaluated KDEd values substantially increased with increasing concentrations in the range above ca. 1.0 M strongly suggested the formation of higher order intermolecular associations instead of dimers. Chemical shifts in the 1H-NMR spectra assigned to (N)CH3 and (5)CH2 protons of NMP and the two (Nα)CH3 and (Nβ)CH3 protons of DMAc dissolved in (d)Bz substantially changed with increasing concentrations due to the formation of intermolecular associations. The states occupied by MONs, DIMs and tetramers (TETs) were evaluated from chemical shift data as a function of the concentrations in the NMP and DMAc systems. Then, the equilibrium constants for the dimer and tetramer formation (2DIM ⇄ TET) were determined to be KNMRd ∼ 1.2 M−1 and KNMRt ∼ 0.2 M−1 for NMP and KNMRd ∼ 0.35 M−1 and KNMRt ∼ 0.25 M−1 for DMAc. The reason for the differences in the equilibrium constants between NMP and DMAc may be related to the difference in motional freedom between the cyclic and open structures. The formation of anti-parallel DIMs and TETs was also confirmed by semi-empirical quantum chemical calculations for both NMP and DMAc molecules.


 Please wait while we load your content...
Please wait while we load your content...